Our Services

What We Provide Best
With 35+years of rich experience as a CONSULTANT, AUDITORS & TRAINERS into Medical Devices, Pharma, Biotech, & Nutraceutical Industries, we aim to deliver
- Commitments to the clients,
- Focusing on transparency,
- High quality standards
- Explore an alliance to get faster approvals across the Globe along with start to end services.
We are based at Thane-Mumbai and providing services across the Globe [USA, EUROPE, CANADA, BRAZIL, ASEAN, BANGLADESH, MENA, GCC, ROW & ACROSS PAN INDIA]
What We Offer
Clinical Trial Clinical Study
Common Technical Document
What We Offer

Regulatory Services
Regulatory approvals are mandatory to legally sell. Given the complex..

Compliances
Regulatory Compilation of all modules CTD-M1, M2, M3, M4, M5 and Global ...

Audits
Audits & Pre-Approval Inspection as per country-specific requirements...

Quality Systems
We provide made to measure quality system solutions that are...

Labelling Management
Labelling is an important aspect of the marketing of a product. Regulatory...

Documentation
Dossier writing from Module 1 to Module 5 for generic and new drug...
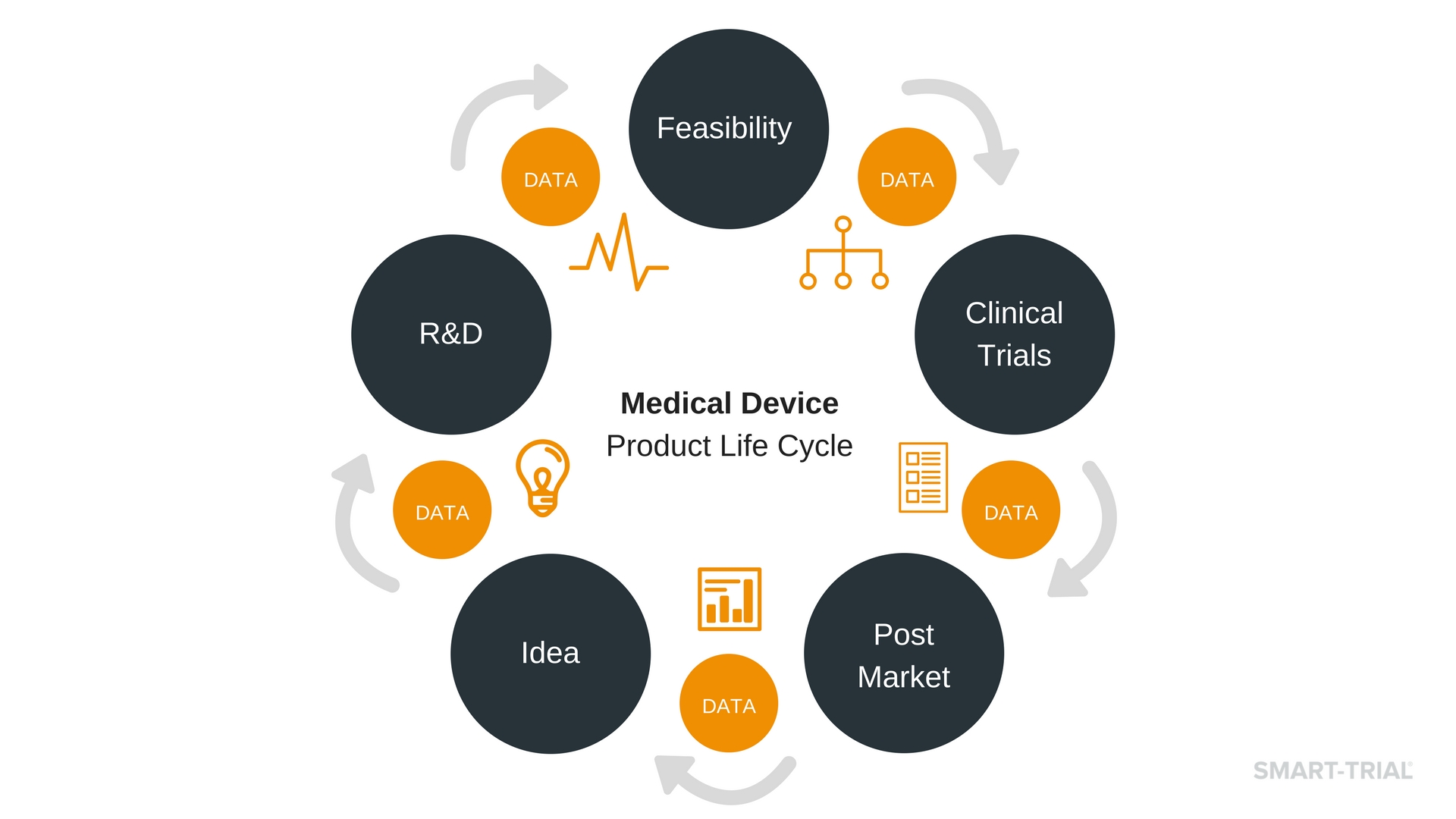
Product Management
Product Life cycle Management i.e., Product variation / amendment...

Validation
We provide made to measure quality system solutions that are tailored...

Training
In House Training and Workshops into RA, QA, GMP, ICH, QbD, Clinical Research, PV...
Clinical Trial Clinical Study
Clinical validation is one of the most important and critical aspects of...
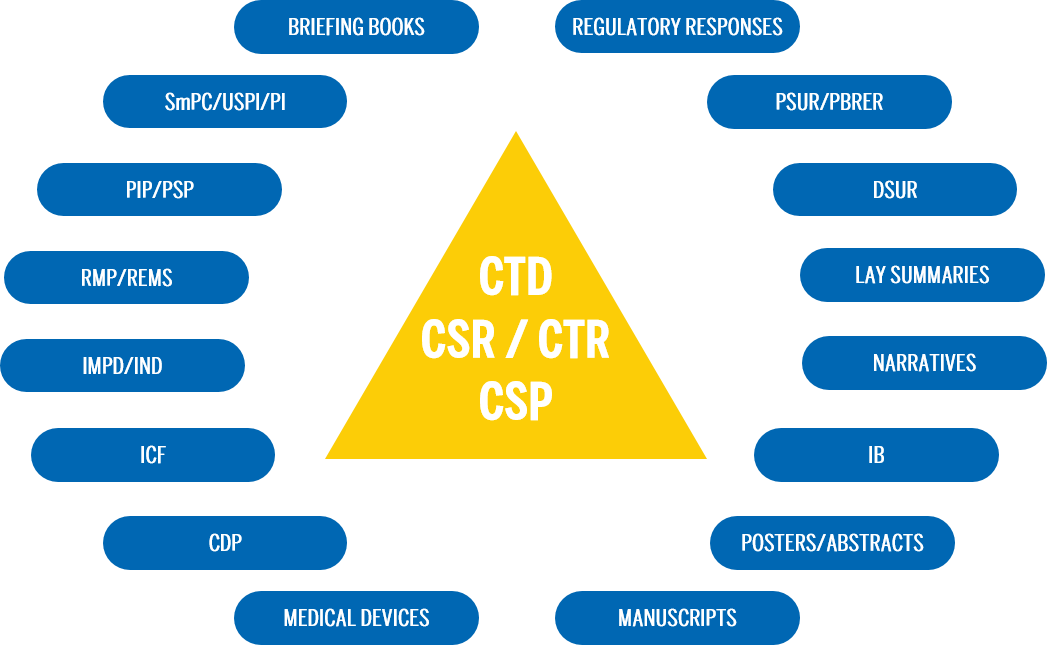
Common Technical Document
MAA CTD for Europe Submission, South Africa-CTD, Australia-CTD...
